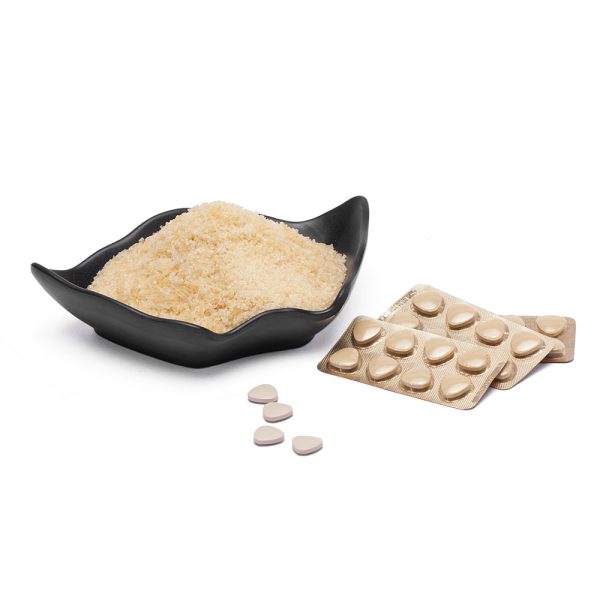
Pharmaceutical Gelatin
The raw material of Kapptai pharmaceutical gelatin is 100% from bovine skins. Kapptai pharmaceutical gelatin has Halal and GPM certificates, and is recognized by Muslim countries. Kapptai pharmaceutical gelatin is widely used in hard capsules, soft capsules, tablets and also Mircro-Encapsulations.
- Description
- Inquiry
Description
There are great customized solutions of Kapptai pharmaceutical gelatin. Kapptai can supply solutions ranging from 120-240 bloom, viscosity 3.0-6.0 mpa.s. We supply pharmaceutical gelatin to many large international pharmaceutical manufacturers. The annual output of our gelatin is 15000 tons, and we can increase the output according to the needs of customers. Kapptai gelatin has ISO22000, HACCP, GMP, Halal and other certificates, which provides a good management system for gelatin production safety and quality control.
- In the application of hard capsules, pharmaceutical gelatin with 200-250 bloom and viscosity of 4.0-4.5 mpa.s is mainly used.
- In the application of soft capsules, pharmaceutical gelatin with 160-240 bloom and viscosity of 3.0-4.0 mpa.s is mainly used
- In the application of tablets, pharmaceutical gelatin with 120-140 bloom and viscosity above 3.0 mpa.s is mainly used.
| Specification | ||||
| Product Name | Pharmaceutical Gelatin | Grade | 140-260Bloom | |
| Test Parameters | Specification | Test Parameters | Specification | |
| Sense Requirements | light yellow to yellow solid granule, powder, flake; odorless ; swell to soften when immerse in water, can absorb 5-10 times of water. | Arsenic(As) | ≤ 1.0 mg/kg | |
| Gel Strength(6.67% 10℃) | 140-260Bloom g (Customerized Solution) | Chromium(Cr) | ≤ 2.0 mg/kg | |
| Viscosity(6.67% 60℃) | 3.0-5.0 mpa·s(Customerized Solution) | Lead(PB) | ≤ 1.0mg/kg | |
| Particle Size | 8-60mesh(Customerized Solution) | Cadmium(Ca) | ≤ 0.5 mg/kg | |
| PH | 4.0-7.2 | Mercury(Hg) | ≤ 0.1 mg/kg | |
| Transmittance | Transmittance 450nm ≥ 70% | Zinc (Zn) | ≤ 30mg/kg | |
| Transmittance 620nm ≥ 90% | Iron (Fe) | ≤ 30mg/kg | ||
| Transparency (5%) | Transmittance 620nm ≥ 90% | Arsenic(As) | ≤ 1.0 mg/kg | |
| Conductivity | ≤ 0.5mS/cm | Chromium(Cr) | ≤ 1.0 mg/kg | |
| Insoluble particles | ≤ 0.2% | Lead(PB) | ≤ 1.5 mg/kg | |
| Sulfur dioxide | ≤ 30 mg/kg | Total Bacteria Count | ≤ 10 CFU/g | |
| Peroxide (H2O2) | ≤ 10 mg/kg | Total Yeasts and molds account | ≤ 10 CFU/g | |
| Loss on drying | ≤ 14.0% | Total Yeasts and molds account | Negative/10g | |
| Ashes | ≤ 2.0% | Salmonella | Negative/25g | |
| Conclusion: China Pharmacopoeia 2020/USP/EP | ||||
Pharmaceutical Gelatin Package: 25kg/bag, PE bags inside and paper bags outside.